Future Prospects of Global Laboratory Proficiency Testing Market


 |
| CAR T Cell Therapy Market |
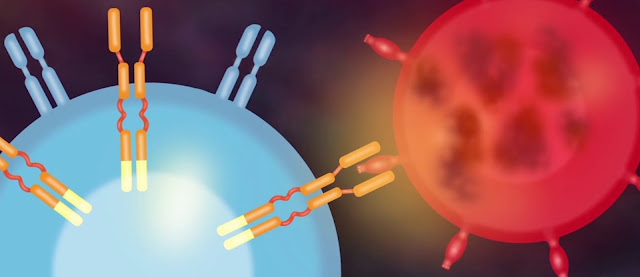
CAR T-cell treatment with chimeric antigen receptors (CAR) is a method of obtaining immune cells known as T cells. CAR T-cell therapy is used to treat lymphomas, leukemias, and multiple myeloma, among other diseases. Many clinical trials for CAR T-cell treatments have been done around the world in order to expand the therapy options for cancer. In 2010, the National Cancer Institute's research team published the first study on CAR T-cell therapy, a new type of cancer treatment.
Increasing approvals for Car T cell therapy products is estimated to drive the global Car T cell therapy market growth during the forecast period. As people become more aware of this novel way of treating cancer, demand for auto T cell therapy products rises. As a result, the industry's major players are working on new items, which is propelling the market forward. For example, the US Food and Drug Administration authorised Yescarta, a cell-based gene therapy, in October 2017 for the treatment of adult patients with certain kinds of large B-cell lymphoma. Yescarta's approval represents a breakthrough in the field of CAR-T cell treatments for cancer patients.
CAR-T
(chimeric antigen receptor T) cell treatment is a viable option. CAR-T cell
structure and manufacturing innovations have resulted in a significant increase
in efficacy, especially with the development of fourth-generation CAR-T cells.
As a result, product efficacy improves, driving market expansion.
Global Car T Cell Therapy Market –
Impact of Coronavirus (COVID-19) Pandemic
As a result
of the COVID–19 outbreak and a reduction of global research effort, many firms
around the world have seen their operations and financial performance
deteriorate. The closing of academic and scientific institutes, as well as
testing facilities, has had a significant detrimental impact. As a result, the
vehicle T cell therapy clinical trials have been pushed back. COVID-19 is a
serious hazard to the health of susceptible patients, such as those who are
immunocompromised. Due to their severely immunocompromised state, induced by
past lymphodepleting immunochemotherapy and CAR-T-cell therapy-related side
effects such as B-cell depletion, hypogammaglobulinemia, and cytopenias,
CAR-T-cell therapy recipients are at a significant risk of poor COVID-19.
Global Car T Cell Therapy Market:
Restraint
CAR T
treatments are notorious for posing special drug development problems. The
market's growth is likely to be hampered by some possible problems linked with
CAR T development. Limited USFDA guidance, production and distribution
logistics, product safety, and so on are all potential problems.
Comments
Post a Comment